What are surfactants |  Diese Seite auf deutsch |
Surfactants are substances you find at many places in your every day life. For instance, soups are surfactants. And certain are an important part of any animalic cell (hence human cells, as well). The basic constitute of the cell membrane are certain surfactants, called lipids.
Now but what are surfactant in detail. You probably know that there are substances the are good water soluble (like salt or sugar) and other are not (like oil) . Scientist call them hydrophilic and hydrophobic, respectively. Now if there were nothing else, but this two groups of substance it would be impossible to clean some cloth with water if the dirty is some kind of oil. Fortunately there is something more. There are other substances called amphiphiles or surfactants. Both names mean roughly the same. Amphiphile means that there are both hydrophilic and hydrophobic. The name stands for surface active and refers to another feature of this substances.
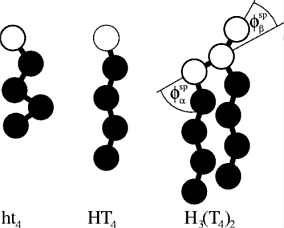 Most surfactants consists of two parts, a hydrophilic headgroup and one to four hydrophobic tail.
The figure aside shows schematic drawing of some surfactants.
This are no real surfactants but the model surfactant is investigated in my
PhD-research.
Most surfactants consists of two parts, a hydrophilic headgroup and one to four hydrophobic tail.
The figure aside shows schematic drawing of some surfactants.
This are no real surfactants but the model surfactant is investigated in my
PhD-research.
As mentioned before, soups (more general tensides) are special surfactants. Since there tails are good in solving oily dirty and the head are good soluble in water the can bridge the gap between hydrophobic and hydrophobic. This happen by forming aggregates with the oil in the center and the tensides around. The tails of the tensides are oriented towards the oil while the head face to the water. In this way the whole aggregate became water soluble, although there is a lot of hydrophobic stuff inside.
For the lipids (the surfactants in the cell membranes) the tails are very hydrophobic.
There you can't hardly solve almost no lipid in water.
Nevertheless if you put lipids in water the lipid will disappear after some time (at least some
portion). This lipid forms some aggregate, either formed spherically or cylindric and planar.
In the first two case one denotes this aggregates as micelles
in the latter as membranes or bilayers.
For all this aggregates the tails face inside the it and the heads sit on the surfaces near the water.
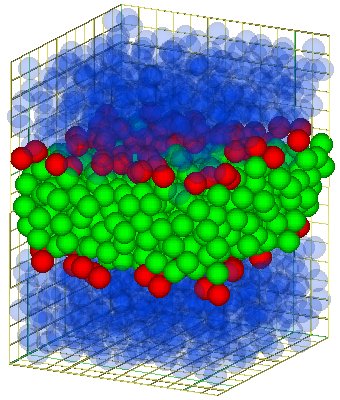 The bilayer consist of two monolayers of parallel oriented lipids. The tails of this two monolayers
face each other and so you get a bilayer (see the the figure aside for a model-bilayer).
For some more figure see the the page about self-assemble.
Due to the large hydrophobicity of the tails this membranes have to form global objects in order
to hide the tails at the edges of the membrane form the water.
This objects are called vesicles.
There diameters is much bigger than the thickness of the membrane. Hence locally on atomic scale
there are more or less planar. Even so the diameter are much bigger the the membrane thickness
there are small in absolute measure, about some micrometers
(The thickness of the membranes is roughly 4 nanometers).
The bilayer consist of two monolayers of parallel oriented lipids. The tails of this two monolayers
face each other and so you get a bilayer (see the the figure aside for a model-bilayer).
For some more figure see the the page about self-assemble.
Due to the large hydrophobicity of the tails this membranes have to form global objects in order
to hide the tails at the edges of the membrane form the water.
This objects are called vesicles.
There diameters is much bigger than the thickness of the membrane. Hence locally on atomic scale
there are more or less planar. Even so the diameter are much bigger the the membrane thickness
there are small in absolute measure, about some micrometers
(The thickness of the membranes is roughly 4 nanometers).
In a way cell are a kind of vesicles. Furthermore even inside of the cell you membranes as boundaries of the cell organells and as small transport-vesicles. However in the cell the membranes aren't purely lipid-membranes. There are a lot of proteins solved in the membrane. The protein are very important for the biology of the cell. Nevertheless the membrane is the fundamental on which the protein act.
In my PhD-thesis I was working on a model system for a kind of generalized surfactants. The points that for relatively large pieces of membrane one has a good theory (basically the Helfrich Hamiltonian). On the other side chemist has good ideas about the basic atomic force in and between single surfactant molecules. However there was a gap between this two ansatzes. It wasn't clear before whether the macroscopic theory will apply even for small membranes with only a few molecule in the bilayer. It turns out the the theory works down to a scale where width and thickness of the piece of membrane are of the the size.